The remedy of enteric bacterial infections utilizing oral bacteriophage remedy may be difficult for the reason that harsh acidic abdomen setting renders phages inactive throughout transit by way of the gastrointestinal tract.
Solid oral dosage types permitting site-specific gastrointestinal supply of excessive doses of phages, e.g., utilizing a pH or enzymatic set off, can be a sport changer for the nascent business attempting to reveal the efficacy of phages, together with engineered phages for intestine microbiome modulation in costly medical trials.
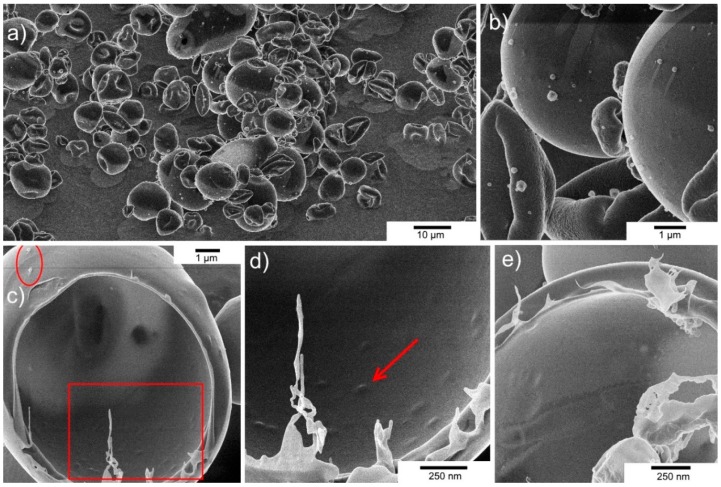
Spray-drying is a scalable, low-cost course of for producing pharmaceutical brokers in dry powder type. Encapsulation of a mannequin Salmonella-specific phage (Myoviridae phage Felix O1) was carried out utilizing the method of spray-drying, using a commercially accessible Eudragit S100® pH-responsive anionic copolymer composed of methyl methacrylate-co-methacrylic acid formulated with trehalose. Formulation and processing situations have been optimised to enhance the survival of phages throughout spray-drying, and their subsequent safety upon publicity to simulated gastric acidity was demonstrated.
Addition of trehalose to the formulation was proven to guard phages from elevated temperatures and desiccation encountered throughout spray-drying. Direct compression of spray-dried encapsulated phages into tablets was proven to considerably enhance phage safety upon publicity to simulated gastric fluid.
The outcomes reported right here reveal the numerous potential of spray-dried pH-responsive formulations for oral supply of bacteriophages concentrating on gastrointestinal purposes.
Biophysical Spandrels type a Hot-Spot for Kosmotropic Mutations in Bacteriophage Thermal Adaptation.
Temperature performs a dominating function in protein construction and perform, and life has developed myriad methods to adapt proteins to environmental thermal stress. Cellular programs can make the most of kosmotropic osmolytes, the merchandise of advanced biochemical pathways, to behave as chemical chaperones.
These extrinsic molecules, e.g., trehalose, alter native water construction to modulate the power of the hydrophobic impact and enhance protein stability. In distinction, easier genetic programs should depend on intrinsic mutation to have an effect on protein stability.
In naturally occurring microvirid bacteriophages of the subfamily Bullavirinae, capsid stability is randomly distributed throughout the phylogeny, suggesting it’s not phylogenetically linked and may very well be altered by way of adaptive mutation. We hypothesized that these phages might make the most of an adaptive mechanism that mimics the stabilizing results of the kosmotrope trehalose by way of mutation. Kinetic stability of wild-type ID8, a relative of ΦX174, shows a saturable response to trehalose.
Thermal adaptation mutations in ID8 enhance capsid stability and cut back responsiveness to trehalose suggesting the mutations transfer stability nearer to the kosmotropic saturation level, mimicking the kosmotropic impact of trehalose.
These mutations localize to and modulate the hydrophobicity of a cavern formation on the interface of phage coat and spike proteins-an evolutionary spandrel. Across a sequence of genetically distinct phages, responsiveness to trehalose correlates positively with cavern hydrophobicity suggesting that the extent of hydrophobicity of the cavern might present a biophysical gating mechanism constraining or allowing adaptation in a lineage-specific method.
Our outcomes reveal that a single mutation can exploit pre-existing, non-adaptive structural options to imitate the adaptive results of advanced biochemical pathways.
